Stem Cell Monitoring
Expanding bone marrow-derived mesenchymal stem cells (BMSCs) for clinical studies presents key challenges:
- Donor variability: Functional differences between batches from different donors.
- Passage-related decline: Reduced proliferation and differentiation potential after successive passages.
- Lack of rapid assays: Few methods provide simultaneous functional characterization and quality assessment, including viability, purity, and potency.
Benefits of xCELLigence RTCA for MSC Functional Monitoring
Sensitive morphology detection: Detect minute changes in cellular morphology triggered by ligand-dependent GPCR activation.
Higher yields with fewer cells: Requires only a small number of cells (≥1,100 per well) for results in a few days, or 10–20K cells per well for faster results.
Improved functional predictions: Rapidly distinguish functional status of undifferentiated vs. differentiated BMSCs.
Overview of GPCR stimulation assay
Upon reaching subconfluency, BMSCs are stimulated with specific GPCR agonists (blue arrow) and the cellular response was detected by the xCELLigence system. (B) A close look at the stimulation response observed in (A) (dotted box). Cell index was normalized against the cell index at the time of treatment (NCI). The response window is calculated as the maximum NCI (NCImax) reached upon treatment for a given time point, divided by the NCI of the negative control (NCInegcntl) at that time point.
Application Note: Functional Assessment of Mesenchymal Stem Cells
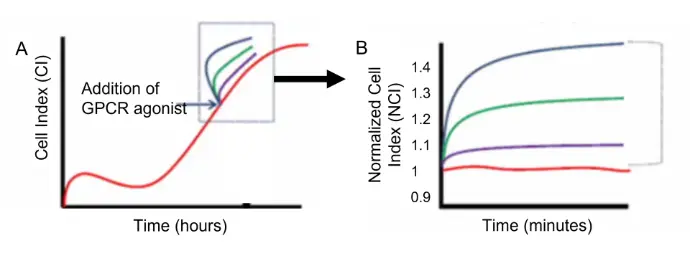 Example of a growth profile of BMSCs as monitored on xCELLigence.
Example of a growth profile of BMSCs as monitored on xCELLigence.Distinct GPCR Profiles in Undifferentiated versus Differentiated BMSCs Provides a Rapid Prediction of Functional Status.
BMSCs from multiple donors were expanded over 12 passages. At selected passages, small cell samples were seeded on E-plates, induced toward adipogenesis, and tracked on xCELLigence to monitor functional capacity in real time.
GPCR Signatures as Rapid QC Markers
BMSCs exhibit distinct GPCR activity profiles that can be leveraged for quick and reliable quality control. Using the xCELLigence GPCR stimulation assay, you can:
- Assess GPCR responsiveness as a direct indicator of MSC functional integrity
- Incorporate quality checks seamlessly during cell expansion
- Use only a small number of cells (≥1,100 cells/well in 96-well plates)
- Obtain actionable results within just a few days—or as early as 48 hours when using higher cell densities (10–20K cells/well)
Quantitative Correlation
Activation of A1AR, P2Y1, and H1 receptors correlates with adipogenic differentiation capacity. This provides a fast, predictive readout of MSC quality and functional integrity.
- Early-passage cells: high GPCR signaling and strong differentiation potential
- Later passages: reduced receptor activity and declining potency
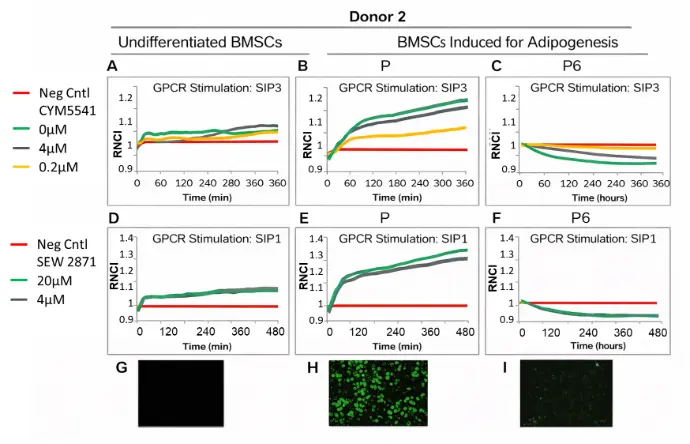
Figure. Donor 2 results (A-I). At 24 hours following adipogenic induction, agonists CYM5541and SEW 2871 were added to undifferentiated control (A, D) or BMSCs induced for adipogenesis at early passage (B, E) or late passage (C, F), to stimulate SIP3 and SIP1 GPCRs, respectively. After 14 days of growth in adipogenic differentiation medium, cells were stained with LipidTox Green Lipid Stain (G-I).
Stem Cell Quality Control Made Simple and Predictive
Large-scale production of bone marrow–derived mesenchymal stem cells (BMSCs) for clinical use requires extensive in-vitro expansion and repeated functional testing. However, this process is often challenged by:
- Donor-to-donor variability that impacts cell performance
- Reduced functional capacity with increasing passages
- A lack of rapid, quantitative assays for assessing viability, purity, and potency in a single workflow
Benefits of xCELLigence RTCA for for MSC QC and Functional Assessment
Ensure batch consistency by tracking integrated changes in cell number, attachment, and morphology.
Maximize yields by requiring fewer cells and enabling analysis at any point during differentiation.
More accurately predict functional capacity, outperforming traditional methods.
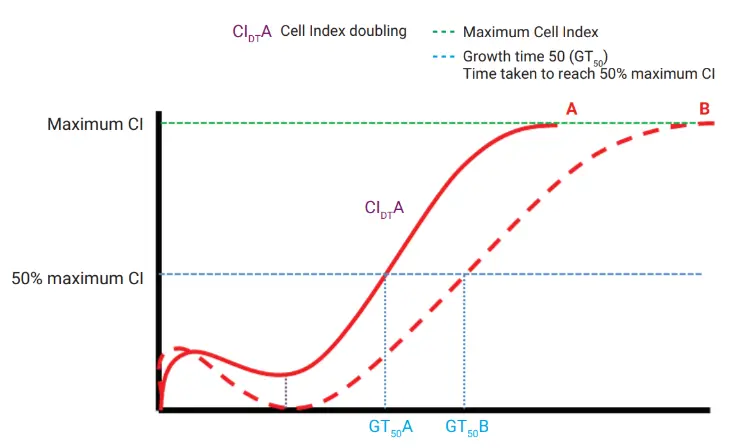
Quantitative Metrics for Confident MSC Characterization
xCELLigence RTCA growth curves provide several powerful parameters:
- CImax – Maximum Cell Index
- GT50 – Time to reach 50% of CImax
- CIDT – Cell Index Doubling Time during logarithmic growth
These metrics deliver a highly quantitative view of MSC quality and performance.
Application Note: Quality Control and Functional Assessment of Mesenchymal Stem Cells
Quality Control Across Donors and Passages
Using xCELLigence RTCA, BMSCs from different donors were tracked across 12 passages in xeno-free media.
Key finding:
Real-time growth profiles revealed significant differences in proliferation potential between donors, clearly visible at passages 2, 6, and 10.
This demonstrates the platform’s ability to detect functional decline and donor variability early and reliably.
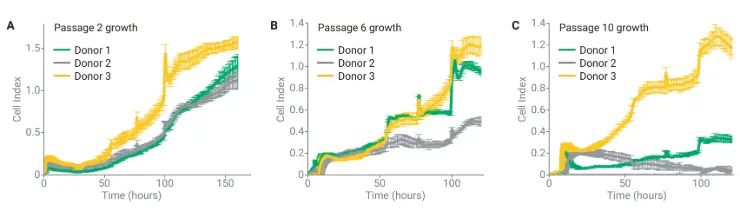
Real-time growth profiles of BMSCs from three donors across 12 passages reveal clear donor-dependent differences in proliferative potential. Shown are representative kinetic curves from passages 2 (A), 6 (B), and 10 (C), generated by seeding cells on E-plates and monitoring them for 10 days.
Quantitative Correlation with Differentiation Potential
GPCR activation in response to A1AR, P2Y1, and H1 receptor agonists correlates closely with adipogenic differentiation potential:
- Early-passage cells (high functional capacity) exhibit strong GPCR signaling and robust receptor expression.
- Mid-to-late passage cells show diminished GPCR activation as they approach senescence, paralleling their decline in differentiation potential.
This measurable correlation provides a rapid, predictive, and quantitative method to assess MSC potency long before traditional endpoint assays can.
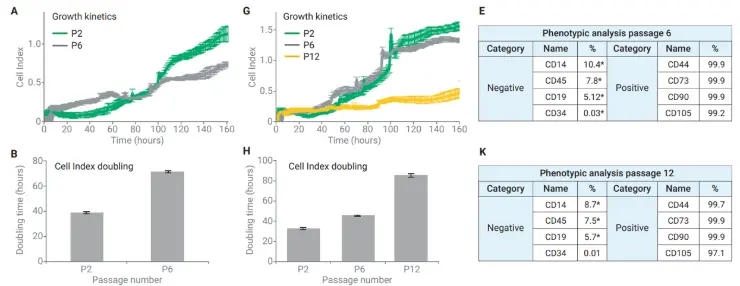
Real-time analysis of BMSCs from donors 2 and 3 shows clear functional decline with increasing passage number. Early vs. late passage growth curves (A, G) reveal reduced proliferative capacity over time. Quantitative metrics, including Cell Index Doubling (B, H) and phenotypic marker profiles (E, K), highlight when cells cross predefined thresholds indicating loss of function.
Stem Cell Differentiation
Differentiating stem cells into specialized somatic cells requires coordinated changes in morphology, metabolism, membrane potential, and cellular responsiveness. With xCELLigence real-time cell analysis, these dynamic transitions can be continuously monitored and quantified - without labels or disruptive handling steps.
Why Use xCELLigence for Stem Cell Differentiation Studies?
Real-time kinetic insights into the full differentiation process.
Label-free and noninvasive, eliminating fixation and staining steps.
Direct correlation of impedance-based readouts with live imaging using E-Plate VIEW.
Sensitive detection of subtle phenotypic and functional changes during lineage commitment.
Monitoring Myoblast Differentiation
Human primary satellite cell–derived myoblasts were seeded in E-Plate L8 (20,000 cells/well). After switching to low-serum differentiation medium (50 hours post-seeding), xCELLigence continuously tracked the fusion of myoblasts and formation of mature myotubes.
Figures courtesy of Dr. Andrea Domenighetti, Northwestern University.

Literature
Application Notes:
Real-Time Quality Control and Functional Assessment of Mesenchymal Stem Cells for Cellular Therapies
Publications:
Stem Cell Monitoring / Stem Cell Quality Control
- Optimization and scale-up culture of human endometrial multipotent mesenchymal stromal cells: potential for clinical application. Rajaraman G, White J, Tan KS, Ulrich D, Rosamilia A, Werkmeister J, Gargett CE. Tissue Engineering Part C Methods. 2013 Jan;19(1):80–92.
- Comparison of long-term retinoic acid-based neural induction methods of bone marrow in human mesenchymal stem cells. Mammadov B, Karakas N, Isik S. In Vitro Cellular and Developmental Biology. 2011 Aug;47(7):484-91.
Stem Cell Differentiation
- Mechanism of 17β-estradiol stimulated integration of human mesenchymal stem cells in heart tissue. Mihai MC, Popa MA, Suica VI, Antohe F, Jackson EK, Simionescu M, Dubey RK.J Mol Cell Cardiol. 2019 Aug;133:115-124.
- Effect of tetrahedral DNA nanostructures on proliferation and osteogenic differentiation of human periodontal ligament stem cells. Zhou M, Liu N, Zhang Q, Tian T, Ma Q, Zhang T, Cai X. Cell Prolif. 2019 May;52(3):e12566.
- Valproic acid stimulates in vitro migration of the placenta derived mesenchymal stem/stromal cell line CMSC29. Al-Sowayan B, Keogh RJ, Abumaree M, Georgiou HM, Kalionis B. Stem Cell Investig. 2019 Feb 13;6:3.
- Loss of myogenic potential and fusion capacity of muscle stem cells isolated from contractured muscle in children with cerebral palsy. Domenighetti AA, et al. Am J Physiol Cell Physiol. 2018. Aug 1;315(2):C247-C257.
- IL-27 regulates the adherence, proliferation, and migration of MSCs and enhances their regulatory effects on Th1 and Th2 subset generations. Xu F, Yi J, Wang Z, Hu Y, Han C, Xue Q, Zhang X, Luan X.Immunol Res. 2017 Aug;65(4):903-912.
- Directed differentiation of skin-derived precursors into functional vascular smooth muscle cells. Steinbach SK, El-Mounayri O, DaCosta RS, Frontini MJ, Nong Z, Maeda A, Pickering JG,Miller FD, Husain M. Arteriosclerosis, Thrombosis, and Vascular Biol. 2011 Dec;31(12):2938-48.
For Research Use Only. Not for use in diagnostic procedures.