Measure Viral Cytopathic Effects (CPE) in Real Time
Accurately tracking viral activity is essential for antiviral drug discovery, vaccine development, and infectious disease research. When viruses infect host cells, they often trigger cytopathic effects (CPE)—including changes in cell size, lysis, fusion, and other hallmark morphological disruptions. When present, these effects provide a powerful, quantifiable readout for virology studies.
Why using xCELLigence RTCA for CPE Assays
Sensitive, high-resolution kinetic data across the entire viral life cycle.
Simple, high-throughput workflows with no agar overlays or labels.
Reduced hands-on time and safer handling of infectious samples.
Greater reproducibility by eliminating subjective visual scoring.
Fully Characterize Antiviral Drug Effects Throughout Infection
The xCELLigence RTCA eSight system delivers multiplexed impedance and live-cell imaging directly inside your incubator. This enables continuous, quantitative monitoring of antiviral activity—capturing key events such as host antiviral pathway activation, apoptosis, and loss of barrier function. Never miss critical timepoints as you follow drug impact from viral entry through cell death.
Multiplex Impedance Data With Live-Cell Imaging
Standard xCELLigence RTCA instruments (SP, MP, HT) provide label-free, noninvasive CPE measurements in 96- or 384-well formats. RTCA eSight adds brightfield and three-color fluorescence imaging, giving you five synchronized data streams from the same cells. This combined perspective strengthens confidence in your interpretations and deepens insight into virus-cell interactions.
Application Note: Detecting and Characterizing Virus Neutralizing Antibodies
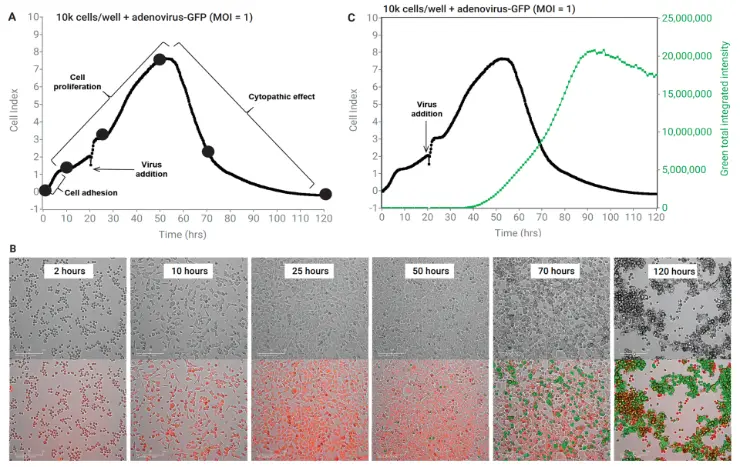
Combining eSight’s impedance- and image-based readouts to track the adenovirus-GFP cytopathic effect. (A) Tracking proliferation and cytopathic effect by impedance. (B) Brightfield, red channel (host nuclei) and green channel (virus-encoded GFP). (C) Impedance- and image-based detection.
Screen and Characterize Virus-Neutralizing Antibodies
Neutralization requires more than antigen binding—it requires blocking infection. xCELLigence impedance assays clearly distinguish neutralizing from non-neutralizing antibodies by measuring their ability to protect host cells across different viral loads. For example, at a low MOI (0.4), a soluble CAR fully prevented adenovirus-GFP–induced cell death, while at a high MOI (222) it showed no protective effect.
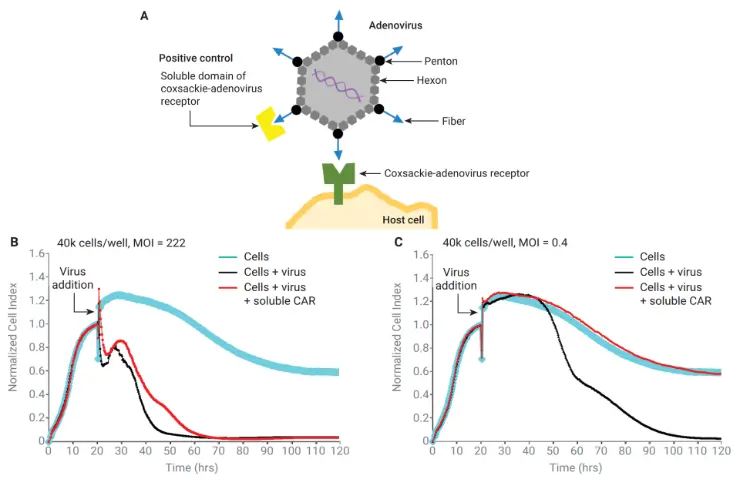
(A) Binding a soluble domain of coxsackie-adenovirus receptor (CAR) to adenovirus to neutralize viral activity. (B,C) Impedance response for 40k cells/well infected at MOI=222 or 0.4 after preincubation with or without soluble CAR.
How It Works
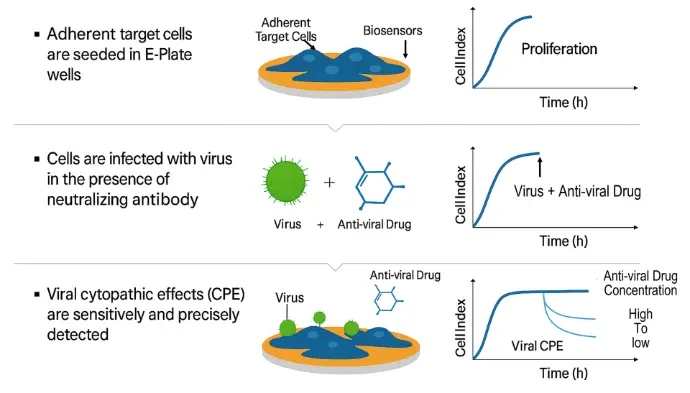
Simple and Robust Antiviral Drug Assay Workflow
xCELLigence RTCA enables fast, sensitive detection of virus-induced cytopathic effects, allowing you to identify and characterize antiviral compounds across the entire viral life cycle. Simply plate target cells, add virus with test compounds at varying concentrations, and let the system automatically capture continuous, label-free kinetic data—eliminating the complexity and delays of traditional plaque assays.
Technical Overview: Screening and Characterizing Antiviral Drugs
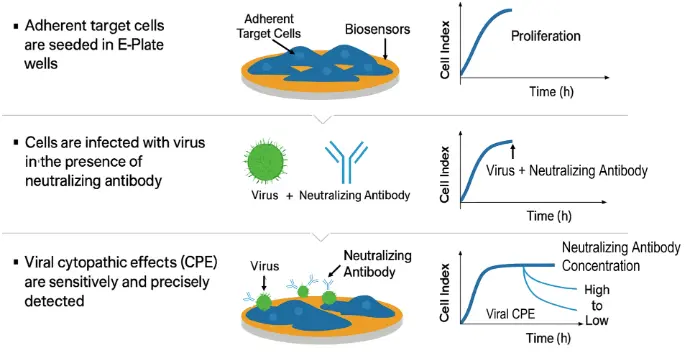
Fast and Efficient Virus-Neutralizing Antibody Screening
Replace labor-intensive PRNT assays with the streamlined xCELLigence RTCA workflow. Plate host cells, add virus together with the test antibody, and the system immediately begins acquiring data—no agar overlays, no manual readouts. Neutralizing activity can be detected within as little as one hour, delivering rapid and highly reproducible results.
Literature
Application Notes
Detecting and Characterizing Virus Neutralizing Antibodies in Real Time
A New Way to Monitor Virus-Mediated Cytopathogenicity
Real-Time Specificity and Potency Assessment of Human Papilloma Virus Specific Engineered T Cells
Handbook
Vaccine and Virology Applications
Technical Overview
Screening and Characterizing Antiviral Drugs in Real Time Using xCELLigence RTCA eSight’s Combination of Impedance and Imaging
For Research Use Only. Not for use in diagnostic procedures.