30-Color Immunophenotyping with NovoCyte Penteon
A 30-color Immunophenotyping Panel of Mice Infected with Influenza Using the Novocyte Penteon Flow Cytometer
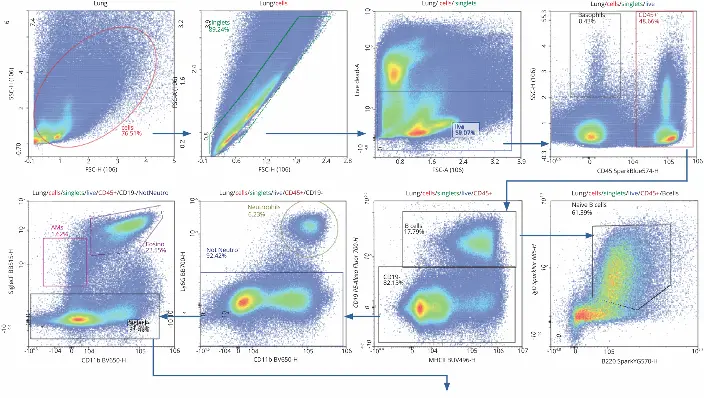
Flow cytometry is a powerful tool for analyzing immune cell subpopulations and gaining a comprehensive view of the immune system. Recent advances now allow multiparametric analysis with many colors simultaneously. In this application note, a 30-color immunophenotyping panel was developed on the five-laser NovoCyte Penteon to profile immune cell distribution in the lung, spleen, and mediastinal lymph nodes of influenza-infected mice.
Key Findings
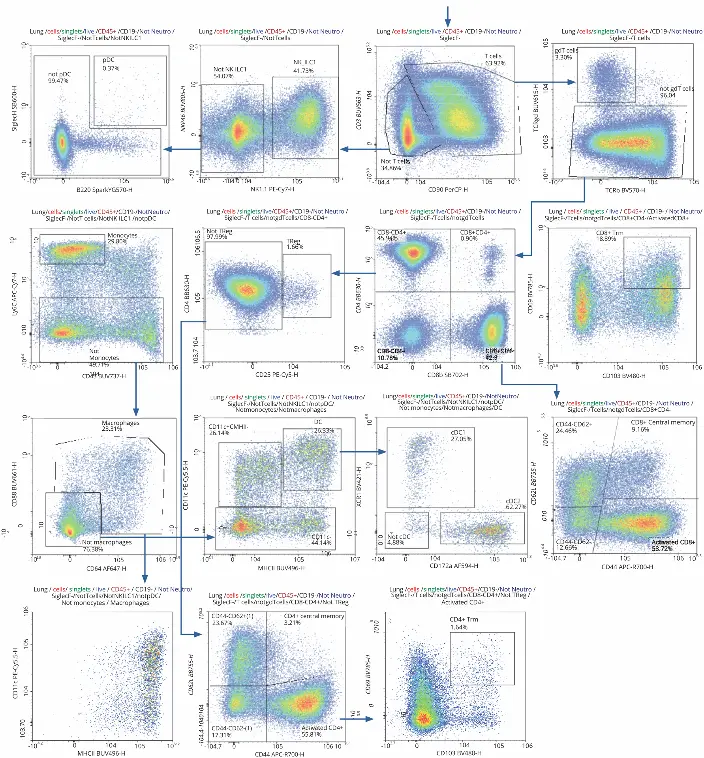
The 30-color panel enabled simultaneous detection of diverse immune cell populations, including T cells, B cells, NK cells, dendritic cells, macrophages, neutrophils, and plasmacytoid dendritic cells.
A consistent gating strategy across organs allowed direct comparison of immune cell distributions.
T cells were most abundant in lymph nodes (90%), followed by lungs (62%) and spleen (54%). Activated CD4+ and CD8+ T cells were dominant in the lung, with CD8+ resident memory T cells (Trm) being notably more frequent than CD4+ Trm cells.
Other cell types, such as neutrophils and pDCs, showed organ-specific distribution patterns, revealing immune heterogeneity during infection.
The system’s ability to perform absolute counts (events/µL) provided additional insight into cell frequencies.
Conclusion
The NovoCyte Penteon enables high-dimensional immunophenotyping in a single acquisition, conserving biological material while offering detailed insights into immune responses. Its sensitivity and 30-color capacity make it a powerful tool for studying complex immune dynamics in infectious disease models.
Revolutionize your research with 30-color immunophenotyping.
Request a free demo today!
Request a Free Demo