Eosinophil Detection and Quantification with NovoCyte
Introduction
Eosinophils play a critical role in immune defense and allergic responses, but traditional identification methods relying on morphology and manual counting can be subjective and inconsistent.
In this study, Dr. Paul Hutchinson (National University of Singapore) collaborated with Agilent (formerly ACEA Biosciences) to develop a flow cytometry-based assay using the NovoCyte Flow Cytometer. This approach enables rapid, accurate, and unbiased detection and quantitation of eosinophils alongside other leukocyte subsets.
Key Findings
Wide dynamic range (7.2 decades): Clear resolution of eosinophils, lymphocytes, monocytes, and granulocytes in the same plot — outperforming competitor systems.
Accurate absolute counts: Achieved via the NovoCyte’s syringe pump without the need for counting beads.
Reliable identification markers: Eosinophils distinguished by FSC/SSC profile, intrinsic autofluorescence, and CCR3 expression.
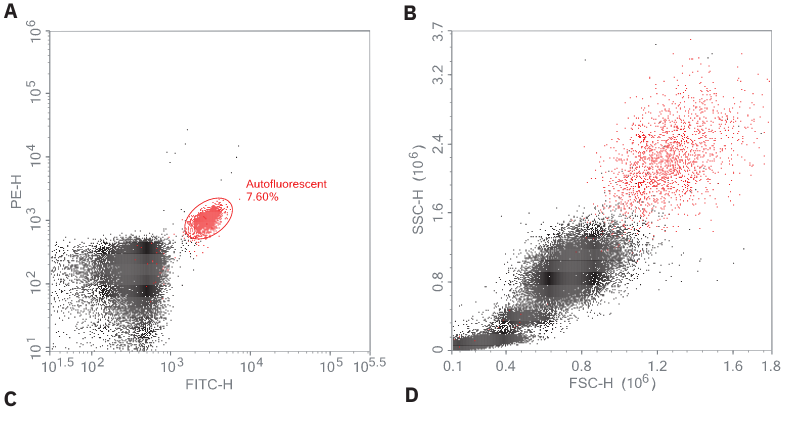
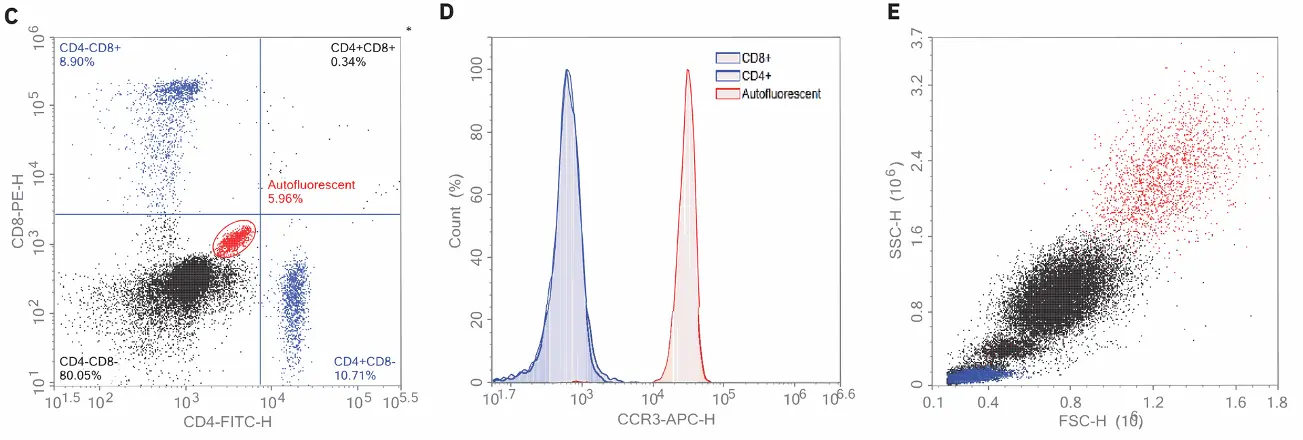
Figure 1: Identification of eosinophils using autofluorescence and CCR3. Human blood was stained with CD4, CD8, and CCR3 antibodies and acquired using the Agilent NovoCyte flow cytometer. Data were then analyzed using the NovoExpress software. Using the backgating technique, the autofluorescent cells detected using FITC/PE (A) were backgated to where eosinophils were expected in the FSC/SSC dot plot (B). Eosinophils were not only confirmed based on their strong intrinsic autofluorescence in the FITC and PE channels (C) but also through their CCR3-positive staining (D). The CD4+ and CD8+ lymphocytes are both negative for CCR3 and all cells are shown backgated to an FSC/SSC plot (E).
Conclusion
The NovoCyte Flow Cytometer provides a robust, high-resolution solution for eosinophil detection in human blood, improving accuracy and efficiency over conventional staining and manual counting methods.
Enhance your eosinophil research with NovoCyte.
Request a free demo today!
Request a Free Demo