mRNA Detection
HPV E6/E7 mRNA Expression Analysis Using the NovoCyte Flow Cytometer
Detection of HPV E6/E7 mRNA provides a direct indication of viral activity, offering greater clinical relevance than DNA testing alone. Using the FLOWSCRIPT HPV E6/E7 assay and the Agilent NovoCyte flow cytometer, researchers demonstrated accurate detection of these oncogene transcripts in both control cell lines and cervical cytology samples.
Key Findings
High accuracy in cell lines: HPV+ HeLa cells showed >95% positivity, while HPV– Jurkat cells remained <1%.
Reliable detection in clinical samples: All HPV+ cervical samples exceeded the 2% positivity threshold, while HPV– samples stayed below it.
Streamlined workflow: Wide dynamic range eliminates PMT adjustments, and optional automation with the NovoSampler Pro minimizes hands-on time.
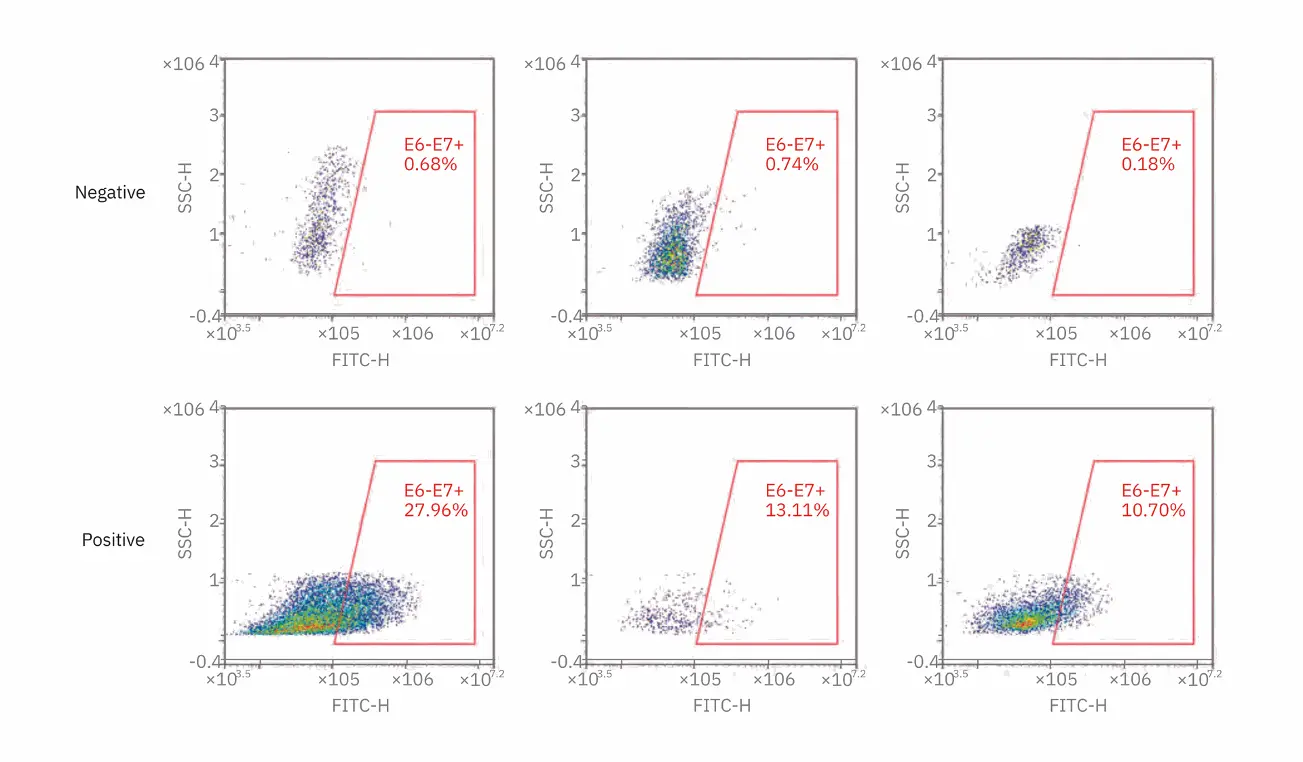
Figure 2. E6/E7 mRNA expression detection in known negative and positive ectocervical cells. Cytology samples were evaluated for the presence of E6/E7 mRNA. Representative positive and negative samples are shown. Threshold is 2% cells within the E6/E7 gate. All HPV negative samples were <2% within the E6/E7 analysis gate, and all HPV positive samples were >2% of the E6/E7 gate.
Conclusion
The NovoCyte flow cytometer enables fast, reliable, and user-friendly single-cell detection of HPV E6/E7 mRNA, improving accuracy and efficiency in HPV research and clinical testing.
Upgrade your HPV mRNA detection workflow with NovoCyte.
Request a free demo today!
Request a Free Demo