Immune Cell Stimulation
Extensive Assessment of Cytokine
Production on the NovoCyte Penteon
Understanding cytokine production is essential for evaluating immune responses to pathogens, autoimmune activity, or new therapeutics.
This application note highlights how the NovoCyte Penteon flow cytometer, combined with BioLegend LEGENDplex bead-based multiplex assays, enables comprehensive cytokine profiling.
In this study, PBMCs from two donors were stimulated with either αCD3 antibody (targeting T cells) or LPS (activating monocytes). Cytokine secretion was measured using the BioLegend LEGENDplex COVID-19 Cytokine Storm Panels 1 & 2 on the NovoCyte Penteon, quantifying 27 cytokines. Additionally, intracellular cytokine staining with surface markers identified the cellular sources of IL-2, IFN-γ, and TNFα in T cells, NK cells, and monocytes.
Key Findings
Quantified 27 cytokines using LEGENDplex COVID-19 Cytokine Storm Panels 1 & 2, providing a broad view of immune activation.
Intracellular cytokine staining identified the frequency and cell type (T cells, NK cells, monocytes, B cells) responsible for cytokine production.
αCD3 stimulation triggered strong T-cell–driven cytokine release (e.g., IL-2, IFN-γ, TNFα, IL-13, IL-4, GM-CSF), while LPS stimulation activated monocyte/macrophage cytokines (e.g., IL-6, IL-1β, IL-18, G-CSF, CCL2, CCL4), each showing distinct cytokine signatures.
Combining bead-based quantification with intracellular stainings delivers both quantitative cytokine profiling and cell-type attribution.
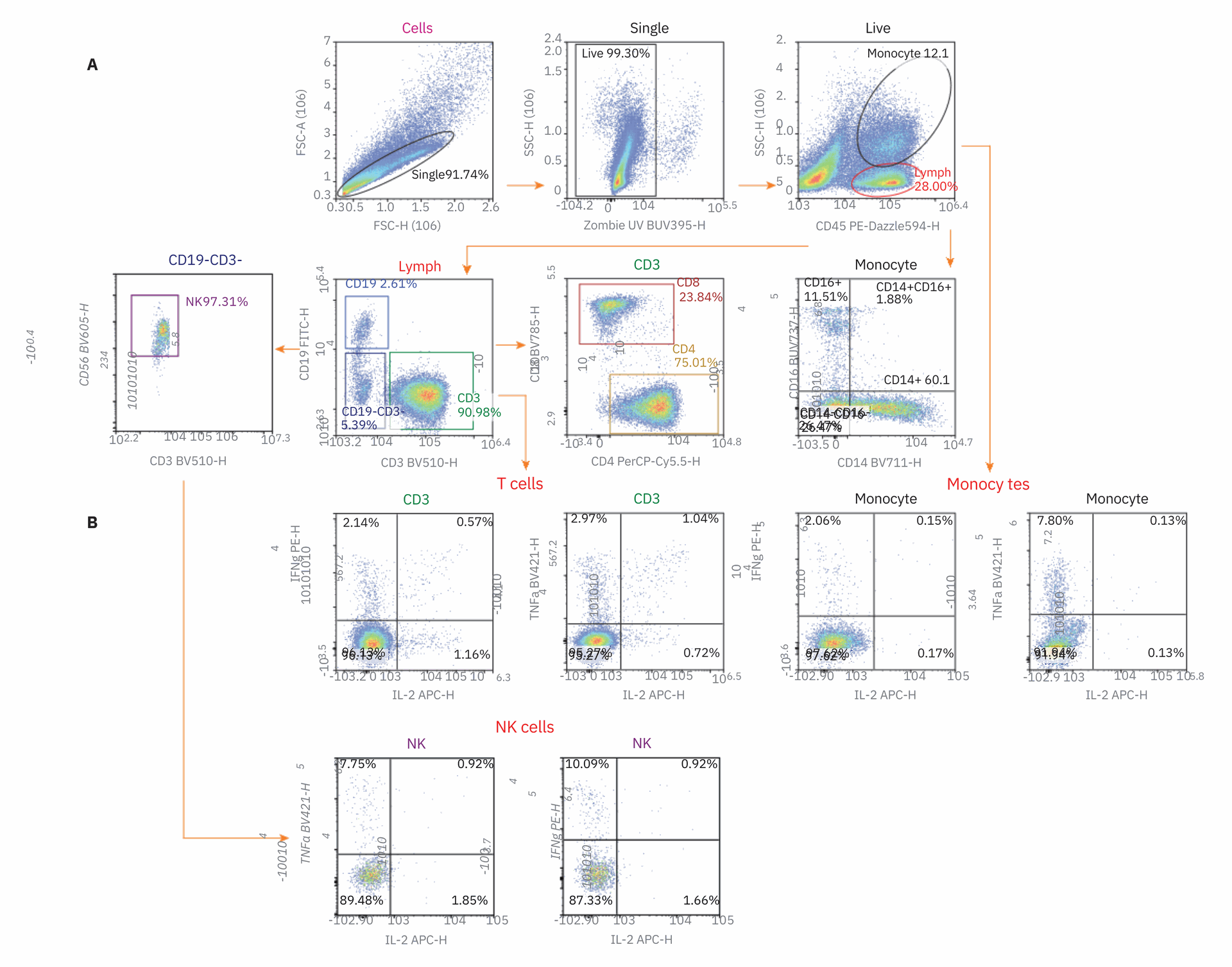
Figure 1. Gating strategy for cell specific cytokine production. Gating hierarchy used for the flow cytometry panel described in Table 1. Isolated peripheral blood mononuclear cells (PBMCs) were stimulated with LPS or added to an anti-CD3 antibody coated plate and cultured from 15 hours with the addition of Brefeldin A. The example shown in this figure was stimulated with CD3 antibody. Cells were then stained with antibody panel and analyzed on the NovoCyte Penteon.
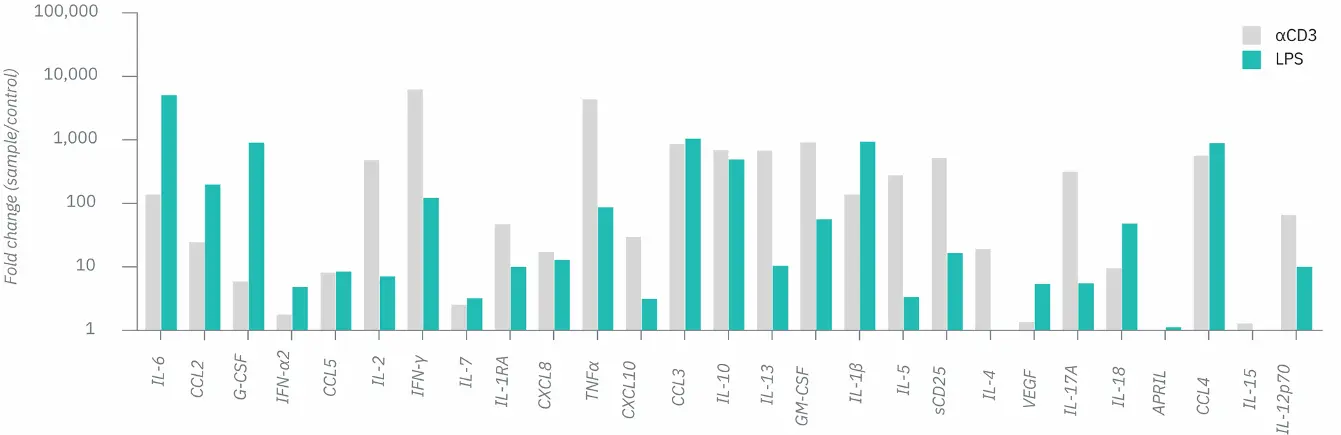
Figure 4. Assessment of the secretion of 27 cytokines with the LEGENDplex COVID-19 Cytokine Storm Panels 1 and 2. Isolated PBMCs were stimulated with LPS (1 ng/mL) PBS control or αCD3/IgG control coated microtiter plates (1 µg/mL). Supernatant was collected at 24- and 48-hour post stimulation. Cytokine production was quantified used the LEGENDplex COVID-19 Cytokine Storm Panels 1 and 2. Here, the fold change in secreted concentration was compared from aCD3 stimulation to IgG control (grey) and LPS to PBS control (green). Any samples that was over the limit of detection was set as the highest value on the standard curve so that fold change could still be calculated.
Conclusion
The NovoCyte Penteon combined with LEGENDplex offers a powerful, high-sensitivity workflow for detailed immune profiling, supporting research into infections, cytokine storms (e.g., COVID-19), and therapeutic safety. This dual strategy enables broad, unbiased cytokine profiling while pinpointing the cellular sources, helping to better evaluate immune responses and mitigate risks such as cytokine storm.
Optimize your immune cell research with high-resolution flow cytometry. Request a free demo today!
Request a Free Demo