Leukocyte Subset Detection
Comprehensive 18-Color Pan-Leukocyte Flow Cytometry Analysis for Immune Surveillance
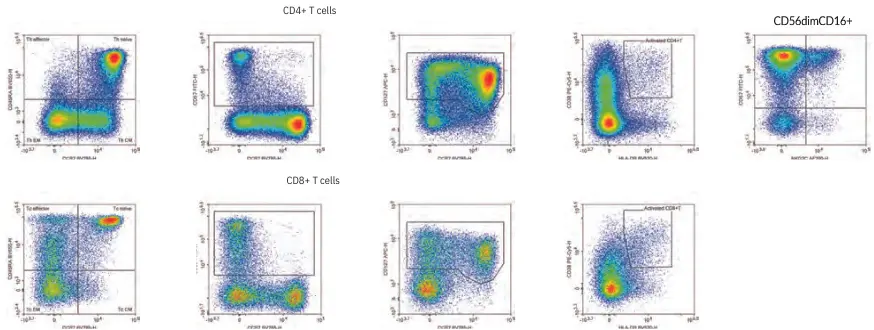
Understanding immune status requires precise monitoring of multiple leukocyte subsets. Using the Agilent NovoCyte Quanteon Flow Cytometer, researchers developed an 18-color immunophenotyping panel to simultaneously identify and quantify a wide range of cell types in human PBMCs — including monocytes, B cells, plasmablasts, CD4+ and CD8+ T cells, regulatory T cells, γδ T cells, NK T cells, NK cells, and dendritic cells.
Key Findings
This optimized panel, based on OMIP-024, enables:
Comprehensive immune profiling from limited sample volumes.
Accurate antibody titration with NovoExpress software to ensure optimal resolution and minimal background.
In-depth analysis of NK and T cell activation and differentiation, critical for evaluating immune responses to vaccines and infections.
Conclusion
The NovoCyte Quanteon empowers high-parameter, high-performance flow cytometry for broad and detailed immune surveillance. This 18-color panel demonstrates how modern cytometry can streamline complex analyses and expand our understanding of immune function.
Upgrade your immune surveillance research with advanced flow cytometry. Request a free demo today!
Request a Free Demo