Alginate Microcarrier
3D Expansion of hiPSCs on Soft Alginate Microcarriers in CERO Bioreactors
Three-dimensional (3D) cell culture systems are transforming stem cell research and bioprocessing. This study demonstrates the successful use of soft alginate microcarriers for expanding human induced pluripotent stem cells (hiPSCs) in CERO suspension bioreactors. These microcarriers provide a physiologically relevant, tunable environment that closely mimics in vivo tissue conditions.
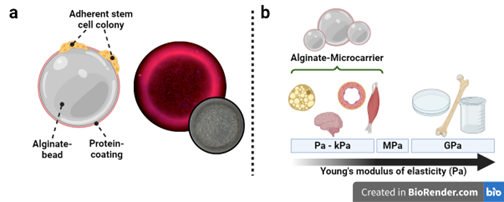
Figure 1: Alginate microcarriers. (a) Right: Schematic drawing of an idealized microcarrier with its elements: alginate beads made from UHV- alginate, the protein coating (e.g. Matrigel™ or Collagen I) for cell attachment. Left: Fluorescence image of alginate microcarrier confluently grown with hiPSC (stained cytoplasm and nuclei), inset: phase contrast image. (b) Alginate microcarriers can accommodate most cell types relevant to soft tissue engineering. Typical labware such as plastic dishes are out of range in terms of the Young’s modulus of tissues.
Key Findings
Ready-to-use alginate microcarriers support efficient hiPSC attachment, growth, and imaging without pre-hydration.
Mechanical properties (2–8 kPa) and size (100–500 µm) can be customized to match specific tissue types.
hiPSC expansion achieved >85% viability and >6×10⁶ cells after four days of culture—independent of microcarrier stiffness.
Cells maintained pluripotency and normal morphology, confirmed by microscopy and FACS analysis.
Soft, transparent microcarriers allow real-time monitoring and easy downstream processing via enzymatic degradation or sieving.
Advantages of UHV-Alginate Microcarriers
Tunable elasticity—from brain-like to heart-like stiffness.
Compatible with multiple coatings (Matrigel™, collagen I, laminin, etc.).
Suitable for various human cell types (hiPSC, hMSC, cardiomyocytes, neural cells, fibroblasts, endothelial cells).
High scalability and low energy demand for bioreactor mixing.
Excellent optical clarity for microscopy.
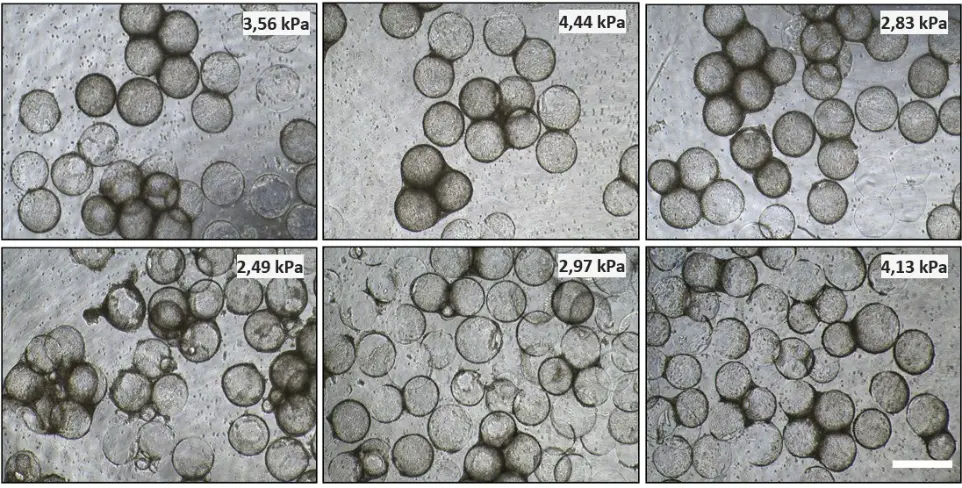
Figure 2: Cell adhesion and proliferation. Representative microscopic images after the fourth 3D cultivation day (96 h, immediately before harvest) of UKBi005-A cell line in the suspension bioreactor on alginate cell scaffolds prepared from differently composed alginate mixtures. The white scale bar corresponds to 500 µm for all images.
Conclusion
UHV-alginate microcarriers are a powerful tool for scalable, high-quality 3D stem cell expansion and differentiation. Their flexibility and compatibility make them ideal for applications in regenerative medicine, tissue engineering, and biopharmaceutical production.
Alginate Microcarriers for Stem Cells.
Request a Free Demo Today!
Request a Free Demo