3D Spheroid Models
Advanced 3D Cell Culture with the CERO 3D Incubator & Bioreactor
The CERO 3D Incubator and Bioreactor transforms 3D cell culture by enabling long-term, physiologically relevant models that overcome the limitations of traditional 2D systems. With exceptional culture stability and high spheroid quality, CERO 3D opens new possibilities in cancer research, virology, angiogenesis studies, toxicology, and advanced therapeutic development.
Key Advantages:
- No necrosis or apoptosis
- Viability maintained for > 80 days
- Improved cellular maturation
- Sustained long-term proliferation
- Highly homogeneous spheroids
- Excellent cell yield
CERO 3D makes complex long-term experiments achievable, offering deep insights into disease mechanisms and therapeutic responses.
Cancer Reserach: New Mechanistic Insights
CERO 3D enables high-fidelity tumor spheroid models for advanced pathway analysis.
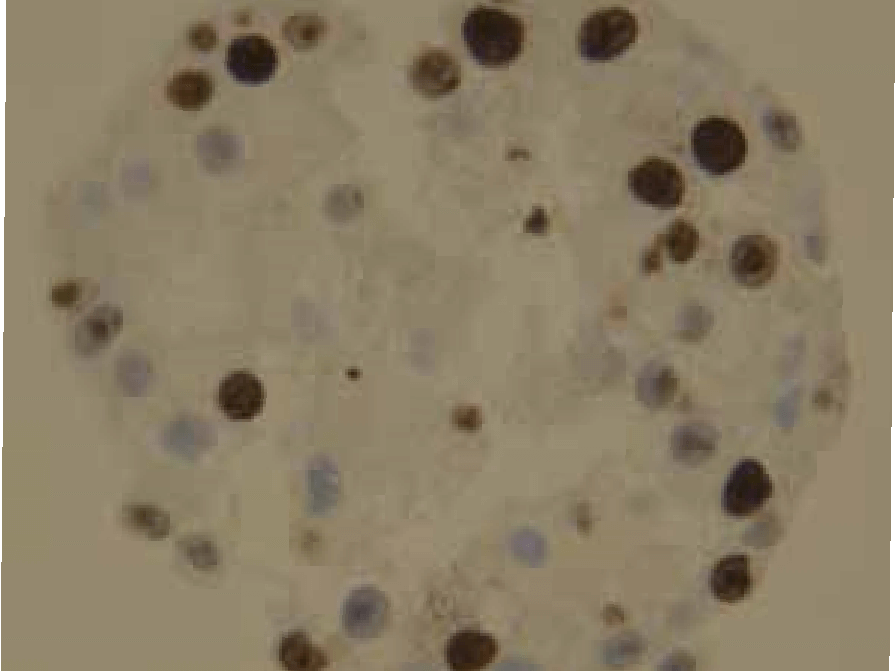
Example: In ovarian cancer research, CERO 3D supported studies on lysophosphatidic acid–driven survival and proliferation mechanisms, providing valuable data for potential therapeutic targets.
Reference: Zhao et. al. (2022) Lysophosphatidic acid suppresses apoptosis of high-grade serous ovarian cancer cells by inducing autophagy activity and promotes cell-cycle progression via EGFR-PI3K/Aurora-AThr288-geminin dual signaling pathways, Front Pharmacol. 2022; 13: 1046269
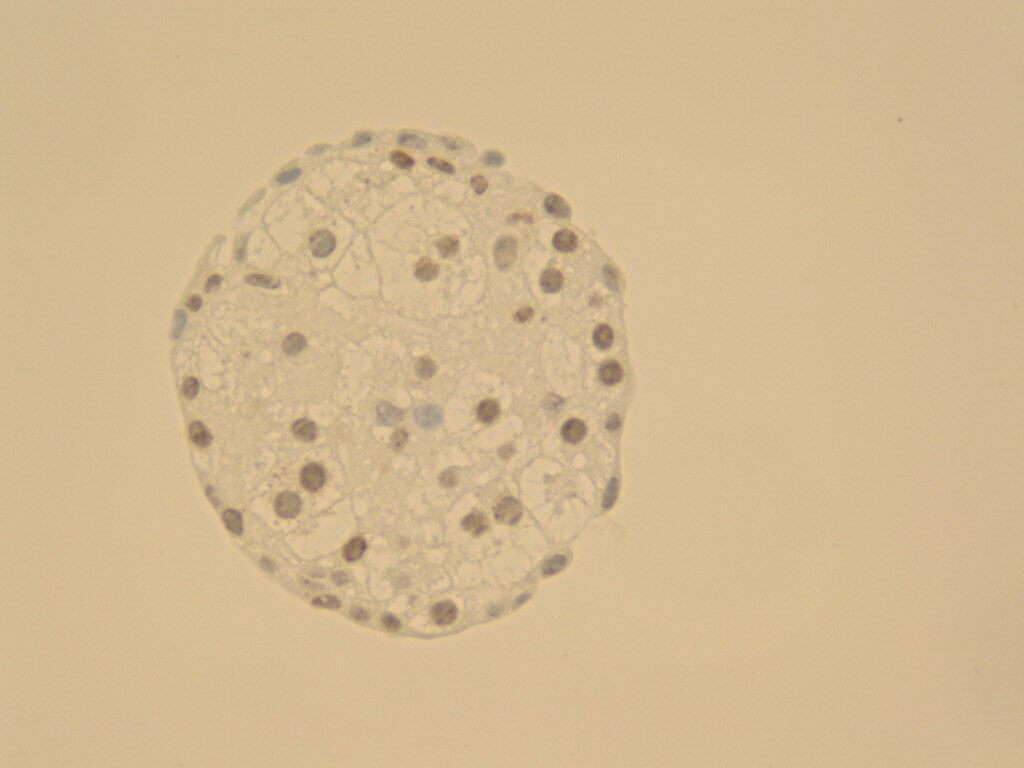
3D Spheroid Models for Hepatitis Virus Research
This example shows spheroids derived from HepG2 cells (hepatocyte cell line) that were cultivated in CERO 3D for more than 80 days:
Enabling In-Vitro Modeling of Angiogenic Disorders
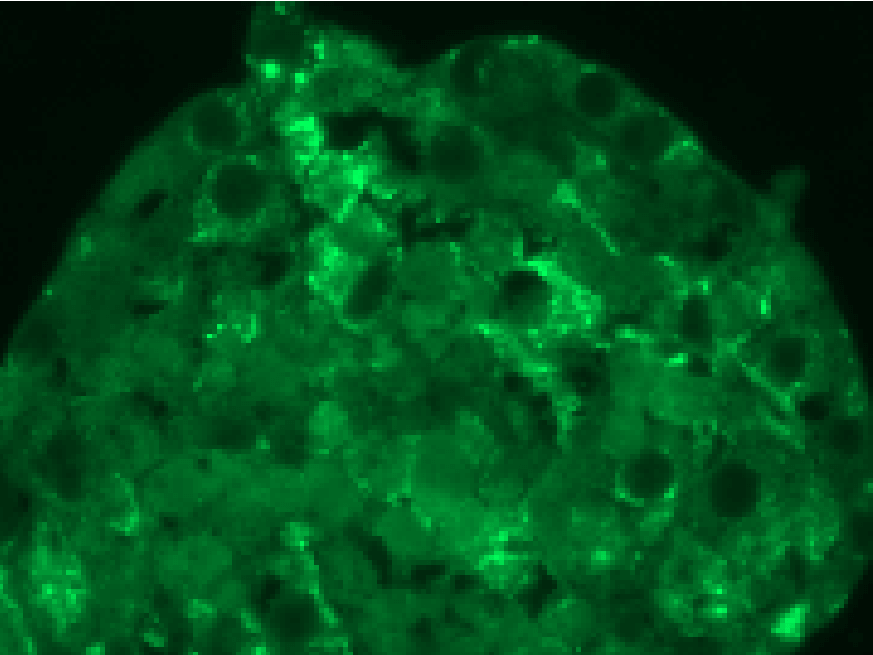
This study leverages CERO 3D's capabilities to create an in vitro model of HHT2 disease, offering a powerful platform for studying angiogenic disorders. By cultivating and differentiating iPSCs with a CRISPR/Cas9-mediated ACVRL1 knockout, researchers can gain valuable insights into the molecular mechanisms underlying HHT2 and potentially accelerate the development of new therapeutic approaches.
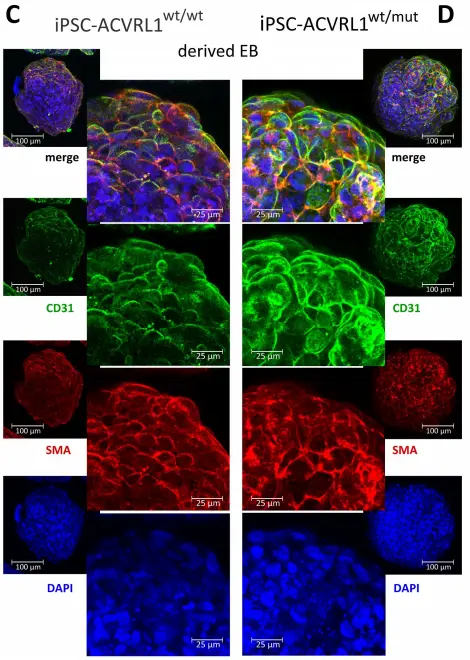
Induction of endothelial differentiation in EBs. Depicted are confocal microscopy images of (A) ACVRL1wt/wt and (B) ACVRL1wt/mut EBs documenting the appearance of CD31+ cells after endothelial induction. Phalloidin was used to highlight the F (filamentous)-actin cytoskeleton, whilst DAPI was used to stain the nucleus. (C,D) Staining of EBs with an antibody directed against smooth muscle actin to detect possible pericyte structures.
Reference: Xiang-Tischhauser et. al. (2023) Generation of a Syngeneic
Heterozygous ACVRL1(wt/mut) Knockout iPS Cell Line for the In Vitro Study of
HHT2-Associated Angiogenesis, Cells. 2023 Jun; 12(12):
1600.
Innovative 3D Tumor Model for Nanoparticle Drug Testing
Utilizing the CERO 3D Incubator & Bioreactor, tumor spheroids were generated from the human colon cancer cell line HT29-MTX-E12. This research led to the development of a microfluidic 3D intestine tumor spheroid model, enabling the testing of functionalized nanoparticles and their permeation across a mucus layer. The model supports the evaluation of nanoparticulate drug delivery systems, with a focus on improving oral drug delivery by enhancing bioavailability and targeting effectiveness.
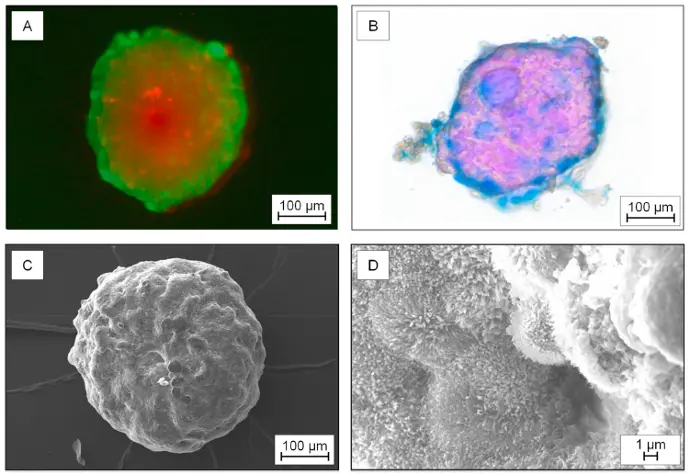
“Characterization of the 3D-tumor spheroids generated from the cell line HT29-MTX-E12. A) Live-dead-staining: Simultaneous fluorescence staining of viable (calcein-AM, green) and dead cells (propidium iodid (PI), red). B) Histological stained spheroid: Nuclear fast red – cell nucleus; Alcian blue – acid mucosubstances and acetic mucins. C) Scanning electron microscopy of a spheroid and D) and scanning electron microscopy display of the microvilli on the surface.”
Reference: Elberskirch et. al. (2021) Microfluidic 3D intestine tumor spheroid model for efficient in vitro investigation of nanoparticular formulations, Journal of Drug Delivery Science and Technology 63 (2021) 102496
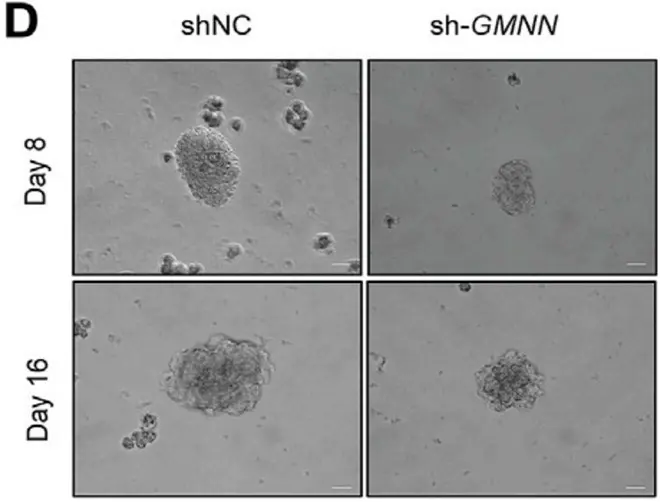
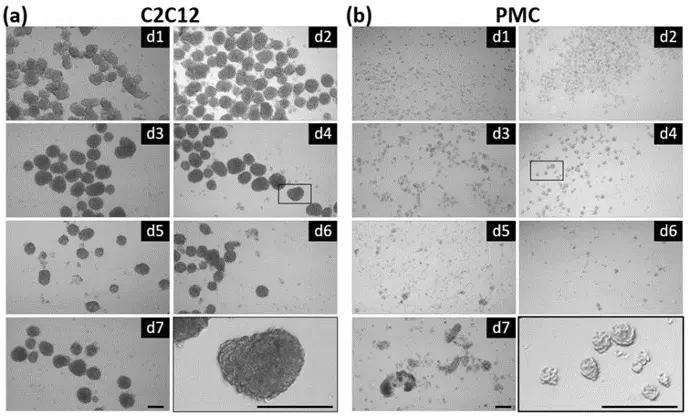
Advanced Production of Myogenic Spheroids
The CERO 3D Incubator and Bioreactorgenerates scaffold-free myospheres. By comparing spheroids derived from primary porcine muscle cells with those from C2C12 cells, the research examines differences in morphology, growth parameters, marker expression, and myogenic potential. This comparison provides valuable insights into the production and development of myogenic spheroids for various applications.
Reference: Stange et. al. (2022) Preparation of Spheroids from Primary Pig Cells in a Mid-Scale Bioreactor Retaining Their Myogenic Potential, Cells. 2022 May; 11(9): 1453.
Revolutionize virology studies with CERO 3D.
Request a free demo today!
Request a Free Demo