Scalable Expansion and Differentiation of iPSCs with CERO 3D
Scalable Expansion of iPSCs
This paper explores the use of induced pluripotent stem cells (iPSCs) in biomedical applications, with a focus on toxicity testing. The authors detail strategies for scalable iPSC production using the CERO 3D suspension culture platform and a benchtop bioreactor. Additionally, they highlight recent advancements in scalable differentiation protocols to generate cardiac, neural, and hepatic lineages, expanding the toolkit for research and therapeutic development.
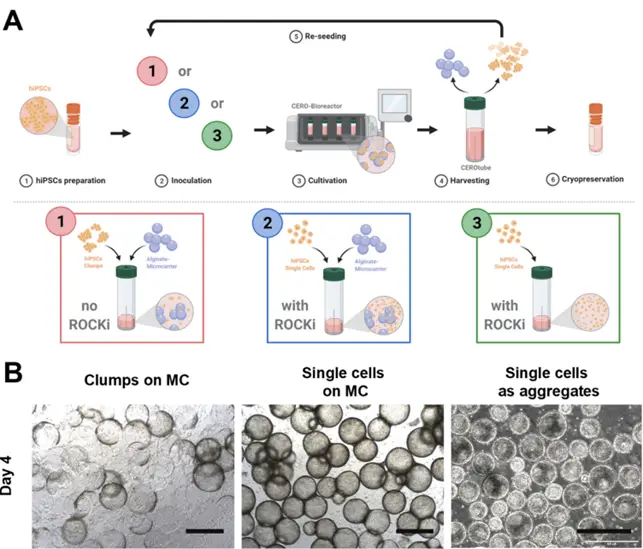
Cultivation and expansion of iPSCs in 3D culture conditions. A: Workflow options of the 3D suspension cultivation. A1: iPSC clumps are seeded on Matrigel™- coated alginate microcarriers. A2: iPSC single cells are seeded on Matrigel™-coated alginate microcarriers in medium supplemented with 10 µM Y-27632. A3: iPSC single cell suspension forms aggregates with ROCK-inhibitor Y-27632 support. B: Morphology of the cells cultured as clumps and single cells on Matrigel™-coated alginate microcarriers (MC) and as aggregates. The cell line UKBi005-A was used for both microcarriers approaches and the cell line BIONi010-C for the aggregates approach. Scale bar = 500 µm.
Reference: Keong Kwok et. al. (2022) Scalable expansion of iPSC and their derivatives across multiple lineages, Reprod Toxicol. 2022 May 17;S0890-6238(22)00068-5.
Scalable Production of Microglia
The CERO 3D Incubator & Bioreactor was employed for the large-scale production of embryoid bodies from human iPSCs and for co-culturing cortical spheroids containing iPSC-derived microglia (iPSdMiG) over periods of 1 to 4 weeks. This research highlights an in vitro differentiation method for producing and cryopreserving iPSdMiG at scale. These microglia displayed a transcriptomic profile similar to adult human microglia, expressed key microglial markers, and demonstrated essential functions, including cytokine secretion, phagocytosis, and ROS production, making them ideal for integration in neural co-culture models.
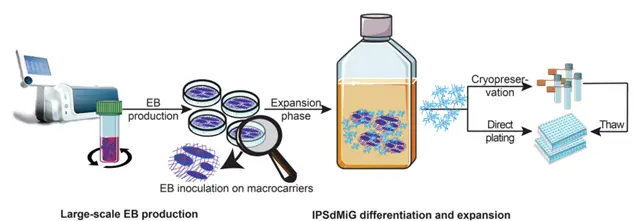
A schematic workflow illustrates the scalable generation of iPSdMiG: EBs are produced and guided toward primitive hematopoietic and early neural precursor lineages, then expanded on mesh macrocarriers. Microglia can be continuously harvested from week 6 to week 12 of differentiation.
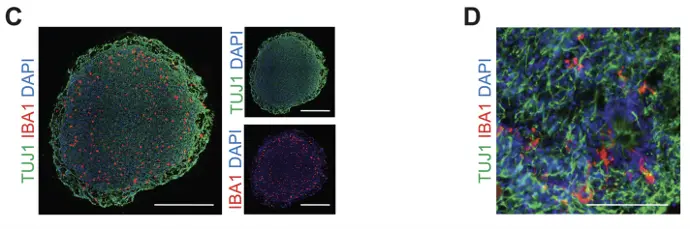
Representative immunofluorescence images show that iPSdMiG (IBA1, red) efficiently migrate into and distribute throughout 3D cortical spheroids (TUBB3, green). Scale bars=500 m (C). Higher-magnification views highlight their characteristic ramified morphology after four weeks of co-culture.Scale bar=100 m (D).
Reference: Mathews et. al. (2022) Reenacting Neuroectodermal Exposure of Hematopoietic Progenitors Enables Scalable Production of Cryopreservable iPSC-Derived Human Microglia, Stem Cell Reviews and Reports
Enabling Efficient and Scalable Stem Cell Therapies
This study demonstrates the successful use of the CERO 3D system cultivating and differentiating human iPSCs into neural lineages. Bulk cryopreservation of iPSCs, followed by expansion and differentiation in CERO 3D bioreactors, offers a promising approach for large-scale production of stem cells for therapeutic applications. This technology has the potential to accelerate the development of innovative, cost-effective stem cell-based therapies.
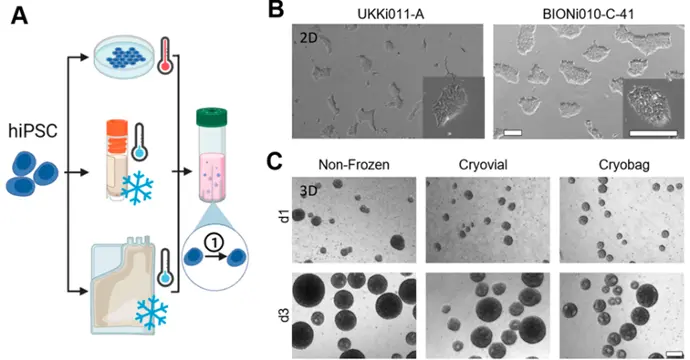
A condensed workflow illustrates the process: the hiPSC lines UKKi011-A and BIONi010-C-41 were cryopreserved at high density using slow-rate freezing—either in cryovials (1 mL, 2 × 10⁷ cells) or cryobags (50 mL, 1 × 10⁹ cells)—and directly inoculated into a CERO 3D suspension bioreactor after thawing. Phase-contrast images show the morphology of both lines before cryopreservation in 2D culture, as well as the formation and growth of UKKi011-A spheroids in the bioreactor on day 1 and day 3 for fresh and cryopreserved samples. Scale bars indicate 500 m.
Reference: Meiser et. al. (2023) Application-Oriented Bulk Cryopreservation of Human iPSCs in Cryo Bags Followed by Direct Inoculation in Scalable Suspension Bioreactors for Expansion and Neural Differentiation, Cells. 2023 Jul; 12(14)
Learn More
The CERO 3D Incubator & Bioreactor enables efficient scale-up, automation, and cost-effective stem cell expansion for biobanking, cell-based drug discovery, toxicity testing, and regenerative medicine. As a dedicated 3D cell culture system, it supports growth in a physiologically relevant environment, far beyond the limitations of traditional 2D culture.
Key advantages:
- Microcarrier-free cultivation
- Stable pluripotency over many passages
- Simple setup and intuitive workflow
- Free-floating, uniform 3D aggregates
- Differentiation into all three germ layers
- Homogeneous iPSC and ESC clusters
Pluripotent stem cells are inoculated as single cells directly into the CEROtube, where they self-aggregate into consistent 3D clusters and can be expanded over multiple passages, requiring only about 2 minutes of hands-on time per day.
The resulting 3D aggregates are immediately suitable for downstream applications, including organoid generation, and 3D or 2D assay development, making CERO 3D an ideal platform for scalable stem cell workflows.
 Human iPSC after expansion in CERO 3D Incubator & Bioreactor tested for pluripotency.
Human iPSC after expansion in CERO 3D Incubator & Bioreactor tested for pluripotency.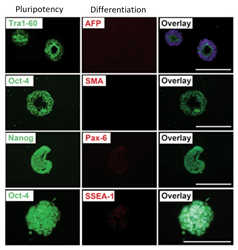
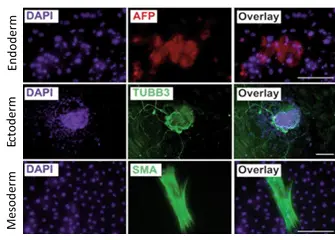
Human iPSC after expansion in CERO 3D Incubator & Bioreactor tested for differentiation in three germ layers.
Reference: Pluripotent stem cells expanded in CERO 3D (former name “BioLevitator”) will maintain pluripotency and can be differentiated into all 3 germ layers, as described by Elanzev et. al. 2015; Biotechnol. J. 2015, 10, 1589–1599:
Optimize your iPSC workflows with CERO 3D.
Request a free demo today!
Request a Free Demo