Cerebral Organoid and Assembloid Culture with Murine aNSCs
Establishing Murine aNSC-Based Protocols Using the CERO Bioreactor
This project marks the first use of the CERO 3D bioreactor for culturing murine adult neural stem cells (aNSCs), developing cerebral organoids, and brain cancer assembloids. While CERO 3D has been tested with human stem cells, we evaluated its performance with murine aNSCs, comparing it to traditional well-plate methods. Results were analyzed for scalability, growth dynamics, and long-term culture stability.
Key Findings
Scalability
Handling larger cell cultures
with less manual work and
greater consistency.
Growth Dynamics
Achieving more controlled
growth and less frequent
splitting and manual labor.
Long-Term Culture
Sustaining organoid growth
over extended periods with
consistent shape and
minimal intervention.

The following highlights showcase the superior performance of the CERO bioreactor in culturing murine aNSCs and cerebral organoids.These key facts demonstrate its advantages in terms of scalability, efficiency, and long-term culture, improving traditional culture methods.
20x Higher aNSCs culture capacity
Supports up to 50 million cells per tube, compared to traditional well-plate methods.
5 days
...for initial neurosphere formation with slow controlled growth and minimal clumping.
2-4x Less frequent aNSCs splitting
Continuous
movement delays
early neurosphere
formation,
reducing the need
for frequent
passaging.
50 % Reduction in manual labor
Fewer media
changes and
less hands-on
time compared
to well-plate
cultures.
> 200 Cerebral organoids
...grown per CERO
tube, ensuring
large-scale
organoid
production.
> 5 Months
...of stable
organoid culture
with consistent
shape and size.
Side-by-Side: CERO 3D Compared to Orbital Shaker/6-well Suspension Plate
Morphology - CERO 3D
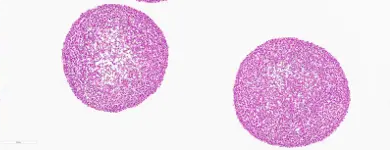
Cerebral organoid after 5-month cultivation
Characterized by even size distribution, round shapes, and proliferating cores.
Morphology - Orbital Shaker

Cerebral organoid after 3-month cultivation
Characterized by uneven size distribution, oval shapes, and apoptotic cores.
Scalability - CERO 3D
- High Capacity: Cultures 200+ organoids per tube, significantly increasing throughput.
- No Fusion: Constant movement prevents fusion of organoids, maintaining controlled growth.
- More Efficient: Larger media volume
reduces the need for frequent media
changes, saving resources and time.
Scalability - Orbital Shaker
- Limited Capacity: Supports ~10-30 organoids per well, but as they grow, organoids tend to touch the bottom, impacting growth.
- Size Variability: Organoids larger than 800 µm often develop uneven sizes and doughnut shapes due to space constraints and inconsistent mixing.
Growth Dynamics - CERO 3D
- Controlled Growth: Slower initial formation allows for even, round organoids, preventing early fusion.
- Consistent Suspension: Organoids stay in suspension, avoiding bottom contact and ensuring uniform growth and nutrient access.
Growth Dynamics - Orbital Shaker
- Uneven Growth: Organoids show size variation and tend to flatten or form doughnut shapes once they grow over 800 µm.
- Bottom Contact: Organoids settle on the well bottom, disrupting uniform growth.
Long-Term Culture - CERO 3D
- Stable for 5+ Months: Organoids remain
viable with proliferating cores, round
shapes, supporting extended culture
without renewal.
Long-Term Culture - Orbital Shaker
- Viability Declines After 3 Months: Larger
organoids develop apoptotic cores and
require replacement.
Revolutionizing
Assembloid Culture
Cerebral Assembloids: A 3D Co-Culture Model
Cerebral assembloids, a co-culture of fused cerebral organoids and cancer spheroids, are an emerging 3R-compliant 3D model, offering an alternative to in vivo studies. Their key advantage lies in the ability to recapitulate both healthy and pathological tissue, closely mimicking conditions observed in cancer patients. However, despite their potential, assembloids have limitations. Both cerebral organoids and spheroids are spherical structures placed together, but their interaction sites are limited, with typically less than 10% of their surface areas interconnected. This does not represent the clinical scenario, where brain cancer is often fully surrounded by healthy brain tissue, creating more complex tumor microenvironment (TME) interactions.
In our proof-of-concept study, we demonstrate that the CERO bioreactor overcomes this limitation, producing assembloid models with over 50% interconnected surface areas, a significant improvement that better reflects the tumor-tissue interactions seen in clinical settings.
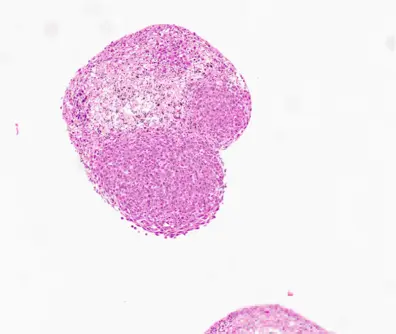
Glioma Assembloid - CERO 3D
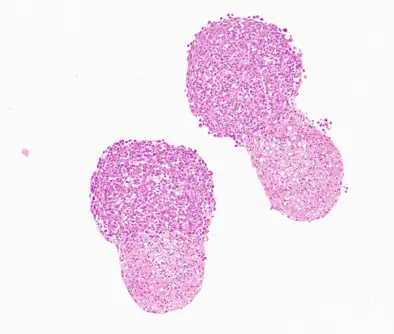
Glioma Assembloid - Orbital Shaker
Procedure Summary
Previously differentiated cerebral organoids were co-cultured with GL261 glioma spheroids (seeded at 10,000 cells per well in a 96-well ULA plate and pre-incubated for 3 days). Organoids and spheroids were placed together in a new 96-well ULA plate, allowing tight cell-cell connections to form over 72 hours. After 72 hours, half of the assembloids were transferred to a CERO tube, while the other half remained in the 96-well ULA plate. After an additional 48 hours (a total of 120 hours), assembloids were fixed, sectioned, and stained using H&E, Ki-67 and Cas-3 for analysis.
Side-by-Side: CERO 3D Compared to 96-Well Ultra-Low Attachment Plates
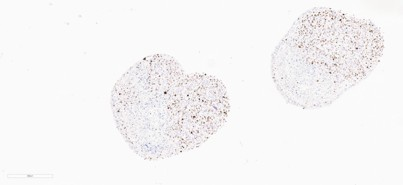
KI67 Proliferation Marker - CERO 3D
KI67 Proliferation Marker - ULA Plate
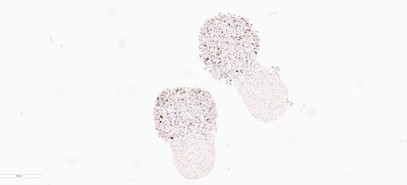
CAS-3 Apoptosis Marker - CERO 3D

CAS-3 Apoptosis Marker - ULA Plate
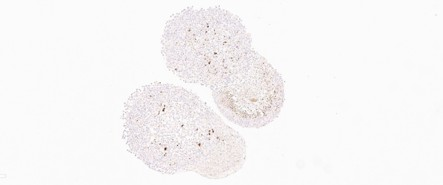
Scalability - CERO 3D
- Enhanced Complexity: The primary strength lies in the greater model sophistication.
- Multiple Assembloids: Several assembloids can be cultured in a single CERO tube.
Scalability - ULA Plate
- Small-Scale Capacity: Supports individual assembloid formation but is limited by the number of wells.
Growth Dynamics - CERO 3D
- Improved Fusion: Promotes over 50% surface interconnection between organoids and tumor spheroids.
- In Vivo Mimicry: Closely mimics tumor invasion, with H&E staining showing cerebral organoids growing around more than half of the tumor tissue.
- Increased Proliferation: Ki-67 staining reveals proliferation in both glioma and organoid tissues.
- Enhanced Viability: Cas-3 staining shows less apoptosis, indicating improved cell viability compared to the ULA plate.
Growth Dynamics - ULA Plate
- Limited Interaction: Less than 10% surface interconnection between organoids and glioma spheroids, leading to weaker tumororganoid interaction.
- Decreased Proliferation: Ki-67 staining shows reduced proliferation in the organoid part of the assembloid over prolonged culture.
- Increased Apoptosis: Cas-3 staining reveals
higher levels of apoptosis in the organoid
tissue compared to the CERO model.
Long-Term Culture - CERO 3D
- Improved Fusion: Enhanced organoidtumor interaction supports better longterm culture.
- Reduced Apoptosis: Lower levels of
apoptosis with proliferating cores in both
glioma and organoid tissues create a more
stable, long-lasting model.
Long-Term Culture - ULA Plate
- Limited Interaction: Organoid-tumor fusion remains weak, affecting the robustness of long-term studies.
- Higher Apoptosis: Increased apoptosis rates, as shown by Cas-3 staining, reduce the model’s viability for extended research.
Special Thank You
We are truly grateful to Dr. Andreas Friese and Dr. Markus Uhrig at OLS OMNI Life Science for giving us the opportunity to work with the CERO. Your support and specialist training have been invaluable and it has been a pleasure collaborating with both of you. We look forward to continuing our joint work, now focusing on brain slice cultivation.
Anna Wolfram, Vanessa Arnold & Prof. Dr. Lisa Sevenich
Optimize your stem cell culture and assembloids workflow with CERO 3D. Request a free demo today!
Request a Free Demo